A Phase 1/2, open-label, multicenter, single-arm trial
Breyanzi®: THE ONE to deliver deep and durable responses in 3L+ CLL or SLL1
Primary endpoints: ORR (including CR and PR) and DOR1
Select secondary endpoints: safety, uMRD, PFS, and OS2
Patients were allowed to receive bridging therapy.1
The study population consisted mainly of patients with difficult-to-treat disease, with 83% having high-risk features* and 51% having bulky disease.1†
Breyanzi demonstrated deep and durable complete responses, with 87.5% of CRs maintained at 18 months1
DOR by best overall response1,3

Primary endpoint:1
- 45% ORR (29/65)
(95% CI: 32.3, 57.5) - 20% CR (13/65)
(95% CI: 11.1, 31.8) - 25% PR (16/65)
(95% CI: 14.8, 36.9)
- 35.3-month mDOR for all responders (95% CI: 12.4, NR)1
- mDOR was not reached for those achieving a CR1
- View an exploratory analysis of mPFS by minimal residual disease status
*Defined as patients having at least 1 of the following features: del(17p), mutated TP53, unmutated IGHV, or at least 3 chromosomal aberrations (complex karyotype).1
†Defined as having at least 1 lymph node lesion with the longest diameter of ≥5 cm.2
3L, third-line; CI, confidence interval; CLL, chronic lymphocytic leukemia; CR, complete response; DOR, duration of response; IGHV, immunoglobulin heavy chain gene; IRC, Independent Review Committee; iwCLL, International Workshop on Chronic Lymphocytic Leukemia™; mDoCR, median duration of complete response; mDoPR, median duration of partial response; mDOR, median duration of response; mPFS, median progression-free survival; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SLL, small lymphocytic lymphoma; TP53, tumor protein 53; uMRD, undetectable minimal residual disease.
Median PFS of 12 months was observed in TRANSCEND CLL 0043‡
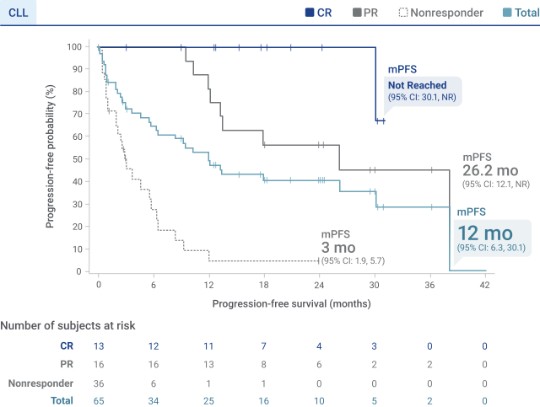
Analysis limitations:
- PFS data are not in the Prescribing Information and should be interpreted with caution
- PFS was a secondary endpoint in TRANSCEND CLL 004 and was not statistically tested in the setting of a single-arm trial. It is not possible to determine if the observed effect is attributable to Breyanzi or to the natural history of the disease2
- The statistical significance of PFS is not known
mPFS was not reached in patients who achieved a CR3
- View an exploratory analysis of mPFS by minimal residual disease status
Median OS of 33.6 months was observed in TRANSCEND CLL 0043§
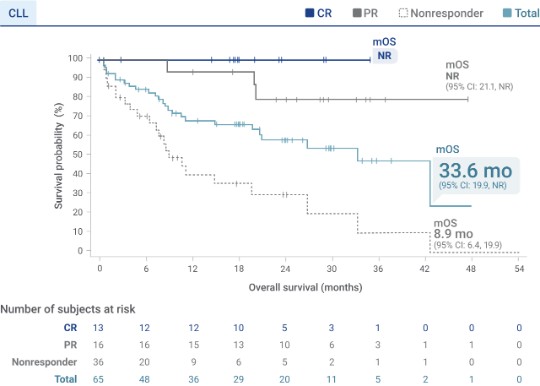
- mOS was not reached in patients who achieved a CR3
- mOS was 33.6 months (95% CI: 19.9, NR)3
Analysis limitations:
- OS data are not in the Prescribing Information and should be interpreted with caution
- OS was a secondary endpoint in TRANSCEND CLL 004 and was not statistically tested in the setting of a single-arm trial. It is not possible to determine if the observed effect is attributable to Breyanzi or to the natural history of the disease2
- The statistical significance of OS is not known
*Defined as patients having at least 1 of the following features: del(17p), mutated TP53, unmutated IGHV, or at least 3 chromosomal aberrations (complex karyotype).1
†Defined as having at least 1 lymph node lesion with the longest diameter of ≥5 cm.2
‡Median follow-up 20.8 months (95% CI: 17.6, 24.4).3
§Defined as the time from Breyanzi infusion to the date of death due to any cause.2
3L, third-line; CI, confidence interval; CLL, chronic lymphocytic leukemia; CR, complete response; DOR, duration of response; IGHV, immunoglobulin heavy chain gene; mOS, median overall survival; mPFS, median progression-free survival; N/R, nonresponder; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SLL, small lymphocytic lymphoma; TP53, tumor protein 53; uMRD, undetectable minimal residual disease.
Breyanzi: THE ONE studied in a broad range of patients, including older patients with more aggressive disease and multiple comorbidities

Screening1
Broad enrollment criteria:
- Adult patients with R/R CLL or SLL
- Had failed at least 2 prior lines of therapy, including a BTKi and a BCL-2i
- ECOG PS 0-1
- LVEF ≥40%
- CrCl ≥30 mL/min
- ALT ≤5x ULN
Enrollment and leukapheresis (N=113)


Breyanzi manufacturing1
Bridging chemotherapy was permitted between leukapheresis and lymphodepleting chemotherapy


Lymphodepletion1‡
FLU 30 mg/m2 and CY 300 mg/m2 × 3 days


Breyanzi infusion1
2 to 11 days after FLU/CY, planned dose of 100 × 106 CAR+ T cells
- Primary endpoints: ORR (including CR and PR) and DOR1
- Select secondary endpoints: safety, uMRD, PFS, and OS2
Patient and disease characteristics (N=65)1§

66
Median age
(range: 49-82)
5 Median number prior therapies
(range: 2-12)
| ECOG PS 0-1 | 100% |
| High-risk features* | 83% |
| del(17p) | 43% |
| TP53 mutated | 45% |
| Complex karyotype∥ | 62% |
| Unmutated IGHV | 45% |
| Bulky disease† | 51% |
Patients studied had a median of 5 prior lines of therapy, plus high rates
of high-risk cytogenetics and bulky disease1
*Defined as patients having at least 1 of the following features: del(17p), mutated TP53, unmutated IGHV, or at least 3 chromosomal aberrations (complex karyotype).1
†Defined as having at least 1 lymph node lesion with the longest diameter of ≥5 cm.2
‡Measurable disease reconfirmed prior to lymphodepletion.2
§The planned dose of Breyanzi was 100 × 106 CAR-positive viable T cells.1
∥Defined as having at least 3 chromosomal aberrations.4
3L, third-line; ALT, alanine aminotransferase; BCL-2i, B-cell lymphoma 2 inhibitor; BTKi, Bruton tyrosine kinase inhibitor; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CR, complete response; CrCl, creatinine clearance; CY, cyclophosphamide; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; FLU, fludarabine; IGHV, immunoglobulin heavy chain gene; LVEF, left ventricular ejection fraction; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; R/R, relapsed or refractory; SLL, small lymphocytic lymphoma; TP53, tumor protein 53; ULN, upper limit of normal; uMRD, undetectable minimal residual disease.
References
- Breyanzi [package insert]. Summit, NJ: Bristol-Myers Squibb Company; 2025.
- Siddiqi T, Maloney DG, Kenderian SS, et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1-2 study. Lancet. 2023;402(10402):641-654. doi:10.1016/S0140-6736(23)01052-8
- Data on file. BMS-REF-LIS-0046. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589-3596. doi:10.1158/1078-0432.CCR-19-3815

