| Number of responders (141/192) |
Median months (95% Cl) |
|---|---|
| DOR | 16.7 (5.3, NR),‡ range§: 0.0±23.5+ |
| DOR if best response is CR |
NR (16.7, NR),‡ range§: 0.7±23.5+ |
| DOR if best response is PR |
1.4 (1.1, 2.2),‡ range§: 0.0±22.8+ |
An open-label, single-arm trial (N=268)
TRANSCEND trial: Largest pivotal trial in 3L+ LBCL, including patients with high-risk disease1,2
Primary endpoints: ORR and safety2
Select secondary endpoints: CR, DOR, and OS2
Bridging therapy prior to receiving Breyanzi® was optional.1
Breyanzi is THE ONE to deliver deep and durable complete response in a one-time infusion, with more than 50% of patients achieving CR1*
Response rates (N=192)1
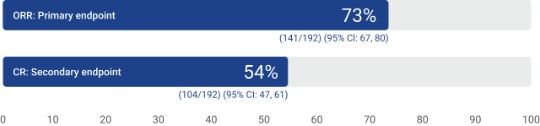
The IRC-assessed overall response rate in the leukapheresed population (N=287) was 59% (95% CI: 53, 64), with a CR rate of 43% (95% CI: 37, 49) and PR rate of 15% (95% CI: 11, 20).1†
Efficacy was established on the basis of CR rate and DOR, as determined by an IRC per the Lugano 2014 criteria.1
DOR rates1
1-month median time to first CR (range: 0.8-12.5 months)1
DOR by best overall response; median DOR: 16.7 months1,3

Of the 104 patients who achieved CR, 68 (65%) had remission lasting at least
6 months, and 64 (62%) had remission lasting at least 9 months1
Median DOR for PR: 1.4 months (95% CI: 1.1, 2.2).1
*Treatment process includes leukapheresis, manufacturing, administration, and adverse event monitoring.1
†Of the 287 patients who underwent leukapheresis and had radiographically evaluable disease, 27 additional patients achieved a response, apart from the responses noted in graph above. These efficacy results include responses that may have been contributed solely by bridging therapy or product outside of the intended dose range or out of specification.1
‡Kaplan-Meier method was used to obtain 2-sided 95% confidence intervals.1
§A plus sign (+) indicates a censored value.1
3L, third-line; CI, confidence interval; CR, complete response; DOR, duration of response; IRC, Independent Review Committee; LBCL, large B-cell lymphoma; NR, not reached; ORR, overall response rate; OS, overall survival; PR, partial response.
Overall survival at 2-year follow-up
mOS: 27.3 months (95% CI: 16.2, 45.6)4
Median follow-up: 29.3 months (95% CI: 26.2, 30.4)4*

51% of all patients were alive at 2 years4
Analysis limitations:
- OS data are not in the Prescribing Information and should be interpreted with caution
- OS was a secondary endpoint in TRANSCEND and was not statistically tested in the setting of a single-arm trial. It is not possible to determine if the observed effect is attributable to Breyanzi or to the natural history of the disease2
- The statistical significance of OS is not known
*Reverse Kaplan-Meier method was used to calculate median (95% CI) of follow-up.4
3L, third-line; CI, confidence interval; CR, complete response; DOR, duration of response; LBCL, large B-cell lymphoma; mOS, median overall survival; N/R, nonresponder; ORR, overall response rate; OS, overall survival; PR, partial response.
TRANSCEND trial1,2

The trial included both patients who had previous stem cell transplants and patients who did not undergo transplant due to ineligibility or other reasons. There was no prespecified threshold for blood counts; patients were eligible to enroll if they were assessed to have adequate bone marrow function to receive LDC.1
- Primary endpoints: ORR and safety2
- Select secondary endpoints: CR, DOR, and OS2
TRANSCEND trial design and patient disposition1
Of 299 patients who underwent leukapheresis:
- 44 (15%) did not receive CAR-positive T cells either due to manufacturing failures (2/44), death (29/44), disease complications (6/44), or other reasons (7/44)
- 204 (68%) received Breyanzi in the intended dose range, of whom 192 were evaluable for efficacy (main efficacy population); 12 were not evaluable due to absence of PET-positive disease at study baseline or after bridging therapy
- 51 (17%) either received Breyanzi outside of the intended dose range (26/51) or received CAR‑positive T cells that did not meet the product specifications for Breyanzi (manufacturing failures [25/51])
Patients were allowed to receive bridging therapy prior to receiving Breyanzi. Optional bridging therapy for disease control included intrathecal chemotherapy or radiation therapy for treatment of CNS lymphoma.1
25 patients were treated in an outpatient setting per physicians’ discretion2
Breyanzi: THE ONE studied in patients you are likely to see in your practice1,2
| Characteristics | (N=268) |
|---|---|
| Median age, years (range) | 63 (18-86) |
| ≥65 years/≥75 years | 42%/10% |
| Large B-cell lymphoma subtype, n (%) | |
| DLBCL, NOS | 53% |
| DLBCL transformed from indolent lymphoma | 25% |
| High-grade B-cell lymphoma | 14% |
| Primary mediastinal large B-cell lymphoma |
7% |
| Follicular lymphoma, Grade 3B |
1% |
3L, third-line; CAR, chimeric antigen receptor; CNS, central nervous system; CR, complete response; CY, cyclophosphamide; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; FLU, fludarabine; LBCL, large B-cell lymphoma; LDC, lymphodepleting chemotherapy; NOS, not otherwise specified; ORR, objective response rate; OS, overall survival; PET, positron emission tomography.
References
- Breyanzi [package insert]. Summit, NJ: Bristol-Myers Squibb Company; 2025.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852.
- Data on file. BMS-REF-LIS-0052. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Abramson JS, Palomba ML, Gordon LI, et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood. 2024;143(5):404-416.

